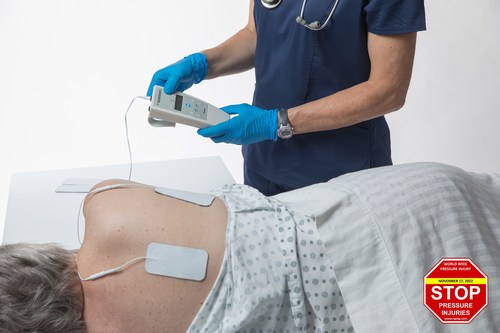
Pilot is designed to test the effectiveness of Prelivia, a neurostimulation device that is designed to protect patients against pressure injuries, which are more common since the beginning of the pandemic
VANCOUVER, BC, Nov. 17, 2022 /PRNewswire/ — To help fight the rising incidence of pressure injuries at long-term care homes, Rehabtronics is starting a pilot with Prelivia, a neurostimulation device designed to protect patients against pressure injuries, also known as bed sores and pressure ulcers.
The pilot was initiated by Responsive Health Management in Ontario, Canada, which is taking a proactive approach to combat pressure injuries (PIs). An increase in PIs can be attributed to multiple factors, many of which could be a consequence of the pandemic including nurse burnout and shortage of qualified medical staff. It can also be due to an increase in long term care residents with complex comorbidities that are often prone to being bedridden.
“We are always concerned about pressure injuries amongst our resident populations and have seen a spike in incidence over the period of the pandemic,” said Marion Godoy, RN, BScN, Senior Nurse Consultant with Responsive Health Management Inc. “Reducing and mitigating the associated risks of infection, pain, and in the most extreme cases, possible death, is our primary concern, so we welcome the opportunity to seize upon new treatment modalities to affect improvement in the area of wound care and pressure ulcers. We hope that Prelivia can assist us not only with increased prevention of PIs, but also with faster healing. Given the human resource crisis in health care, it’s more important than ever to manage our nurses’ time.”
It takes a caregiver only minutes a day to apply electrodes to a resident’s skin in the area of risk and then to monitor the resident throughout the shift. Once activated, muscles are stimulated to contract every ten minutes to maintain healthy blood flow to the site. Studies show that Prelivia increases tissue oxygenation by 28%, which in-turn decreases pressure-induced tissue damage by 80%. It is painless and can be used continuously, 24 hours a day.
“It’s an honor to work with the innovative and proactive team at Responsive Health Management to prove that Prelivia can help reduce the incidence of, and the suffering caused by, pressure injuries while also easing the burden on nursing staff,” said Dr. Rahul Samant, Chief Executive Officer of Rehabtronics, the medical technology company that developed Prelivia. “We are confident that this study will show our painless and economical device fits seamlessly into existing patient treatment workflows and is a valuable addition to any pressure injury prevention program.”
Responsive currently uses a three-step system to prevent pressure injuries, which incorporates cleansers, moisturizers and skin nourishment creams. Currently, good nutrition, skin care and turning residents to decrease pressure on areas at risk, are considered the most important pressure injury prevention strategies.
Prelivia is the first innovation in 70 years aimed at protecting patients from pressure injuries affecting millions of people globally every year. Prelivia is cleared by the U.S. Food and Drug Administration to promote healthy blood circulation and maintain tissue oxygenation in people who are bedridden or chair bound. Health Canada is currently reviewing an application from Rehabtronics.
Responsive is the first long term care facility in Canada to test Prelivia by adding the device to their standard of care. The study is approved by the Independent Review Board.
Responsive Group is a recognized leader in managing senior living environments. Their commitment to excellence continues to ensure their role as creators of practical and innovative processes, programs and partnerships. The company is known for its hands-on approach, which has resulted in the development of reliable processes and time-proven solutions to difficult challenges. https://responsivegroup.ca/.
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003 as a spinoff from the Neuroscience Institute of the University of Alberta, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. Prelivia, the company’s newest product, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices help people recover movement after central or peripheral nervous system injury or disease. www.rehabtronics.com.
Media Contact:
Neena Rahemtulla
604-345-1646
348894@email4pr.com
SOURCE Rehabtronics Inc.